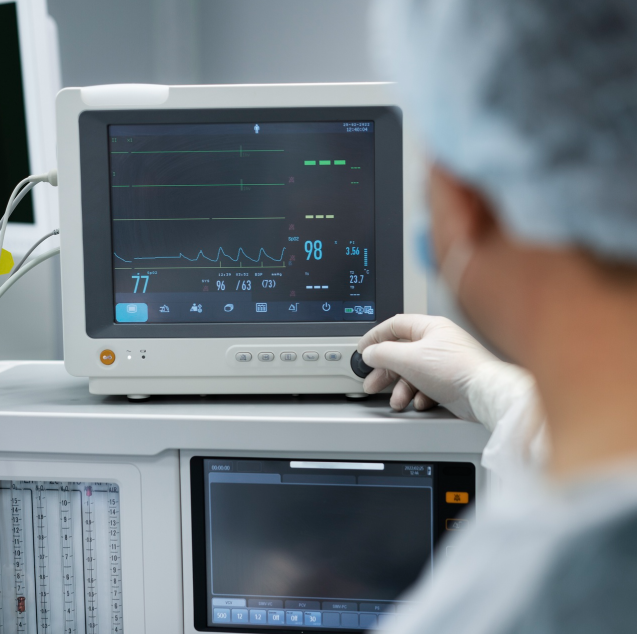
Revolutionizing the dynamics of modern health whilst catering to the needs of patient care through innovative solutions, the medical devices sector has emerged as one of the most important segments in the Indian healthcare infrastructure context. In recent years, the sector has gained momentum through comprehensive R&D in contemporary medicare. A sunrise sector, the market size of the industry is currently $11 Bn, capturing 1.5% of the global market. To accelerate the patient-centric approach with a vision to lead in the manufacturing and innovation of medical devices, the Indian government approved the Medical Devices Policy, which envisages a global share of 10–12% in the next 25 years.
To incentivize the manufacturing of medical devices within the country, the Production-Linked Incentive Scheme for medical devices was also launched, offering financial incentives on incremental sales, and opening up opportunities for both domestic and international players to set up and expand their production capabilities in India.
With India's continued efforts to increase its share in global supply chains, its import dependency on medical devices was also reduced for the first time in 2023. With an enhanced ecosystem and innovation infrastructure, the Indian Medtech sector is poised to grow rapidly, with the recent inauguration of 13 greenfield plants that aim to achieve self-reliance and enhance the indigenous development of cancer-care equipment like linear accelerators for radiotherapy and evolved coronary stents. The global COVID-19 pandemic has made India's role more prominent, with the country being the second-largest manufacturer of Personal Protective Equipment (PPE) kits. Recognizing India as an important growth market, global companies are boosting their R&D initiatives in India. The new R&D centre of Siemens Healthineers in Bangalore has been engaged in developing products for Southeast Asia, Africa, Eastern Europe, and South America, and the rapid adoption of technologies like 3D printing in line with global trends is evident from the inauguration of a 3D bioprinting facility for the development of artificial organs at Andhra Pradesh MedTech Zone. Through the conducive environment of technological innovations and advancements, backed by government policies to enhance support for the industry, the medical device sector is witnessing a transformative change.